How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next
Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”
For Kids with Progeria, New Therapies May Offer Revolutionary Hope for a Longer Life
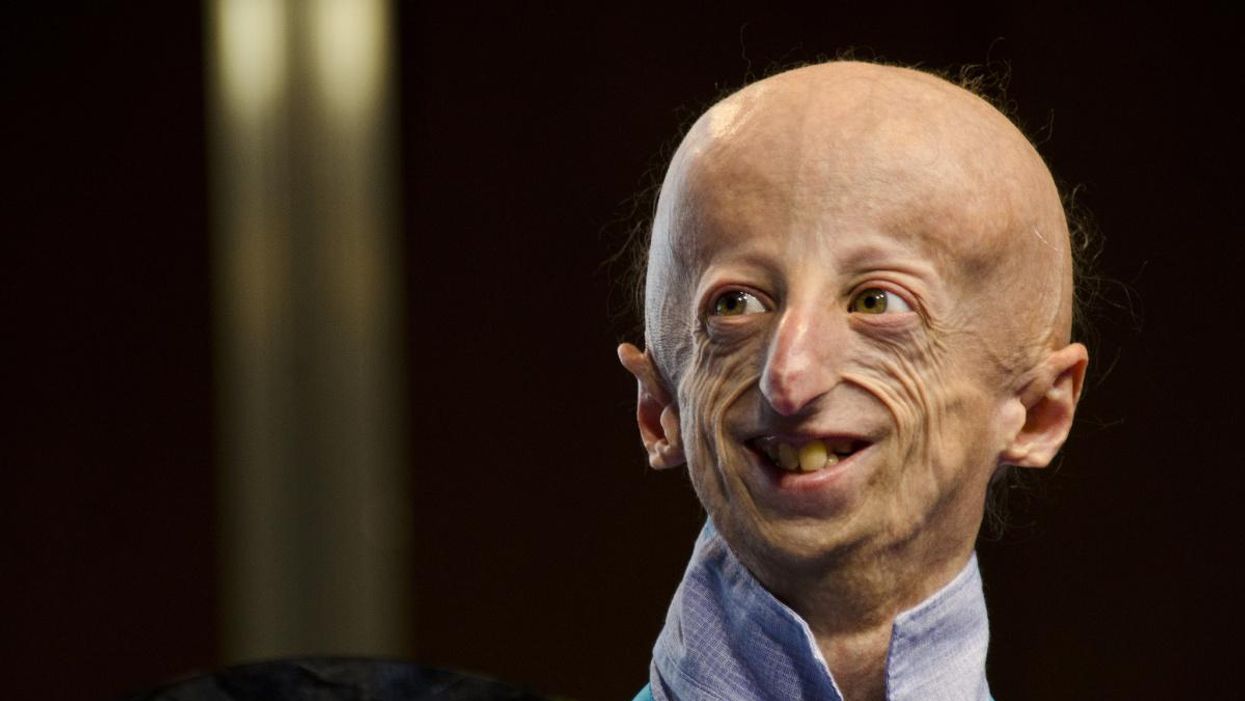
Sammy Basso is one of around 400 young people in the world with progeria.
Sammy Basso has some profound ideas about fate. As long as he has been alive, he has known he has minimal control over his own. His parents, however, had to transition from a world of unlimited possibility to one in which their son might not live to his 20s.
"I remember very clearly that day because Sammy was three years old," his mother says of the day a genetic counselor diagnosed Sammy with progeria. "It was a devastating day for me."
But to Sammy, he has always been himself: a smart kid, interested in science, a little smaller than his classmates, with one notable kink in his DNA. In one copy of the gene that codes for the protein Lamin A, Sammy has a T where there should be a C. The incorrect code creates a toxic protein called progerin, which destabilizes Sammy's cells and makes him age much faster than a person who doesn't have the mutation. The older he gets, the more he is in danger of strokes, heart failure, or a heart attack. "I am okay with my situation," he says from his home in Tezze sul Brenta, Italy. "But I think, yes, fate has a great role in my life."
Just 400 or so people in the world live with progeria: The mutation that causes it usually arises de novo, or "of new," meaning that it is not inherited but happens spontaneously during gestation. The challenge, as with all rare diseases, is that few cases means few treatments.
"When we first started, there was absolutely nothing out there," says Leslie Gordon, a physician-researcher who co-founded the Progeria Research Foundation in 1999 after her own son, also named Sam, was diagnosed with the disease. "We knew we had to jumpstart the entire field, so we collected money through road races and special events and writing grants and all sorts of donors… I think the first year we raised $75,000, most of it from one donor."
"We have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body."
By 2003, the foundation had collaborated with Francis Collins, a geneticist who is now director of the National Institutes of Health, to work out the genetic basis for progeria—that single mutation Sammy has. The discovery led to interest in lonafarnib, a drug that was already being used in cancer patients but could potentially operate downstream of the mutation, preventing the buildup of the defective progerin in the body. "We funded cellular studies to look at a lonafarnib in cells, mouse studies to look at lonafarnib in mouse models of progeria… and then we initiated the clinical trials," Gordon says.
Sammy Basso's family had gotten involved with the Progeria Research Foundation through their international patient registry, which maintains relationships with families in 49 countries. "We started to hear about lonafarnib in 2006 from Leslie Gordon," says Sammy's father, Amerigo Basso, with his son translating. "She told us about the lonafarnib. And we were very happy because for the first time we understood that there was something that could help our son and our lives." Amerigo used the Italian word speranza, which means hope.
Still, Sammy wasn't sure if lonafarnib was right for him. "Since when I was very young I thought that everything happens for a reason. So, in my mind, if God made me with progeria, there was a reason, and to try to heal from progeria was something wrong," he says. Gradually, his parents and doctors, and Leslie Gordon, convinced him otherwise. Sammy began to believe that God was also the force behind doctors, science, and research. "And so we have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body," he says.

Sammy Basso and his parents.
Courtesy of Basso
Sammy began taking lonafarnib, with the Progeria Research Foundation intermittently flying him, and other international trial participants, to Boston for tests. He was immediately beset by some of the drug's more unpleasant side effects: Stomach problems, nausea, and vomiting. "The first period was absolutely the worst period of my life," he says.
At first, doctors prescribed other medicines for the side effects, but to Sammy it had as much effect as drinking water. He visited doctor after doctor, with some calling him weekly or even daily to ask how he was doing. Eventually the specialists decided that he should lower his dose, balancing his pain with the benefit of the drug. Sammy can't actually feel any positive effect of the lonafarnib, but his health measurements have improved relative to people with progeria who don't take it.
While they never completely disappeared, Sammy's side effects decreased to the point that he could live. Inspired by the research that led to lonafarnib, he went to university to study molecular biology. For his thesis work, he travelled to Spain to perform experiments on cells and on mice with progeria, learning how to use the gene-editing technique CRISPR-Cas9 to cut out the mutated bit of DNA. "I was so excited to participate in this study," Sammy says. He felt like his work could make a difference.
In 2018, the Progeria Research Foundation was hosting one of their biennial workshops when Francis Collins, the researcher who had located the mutation behind progeria 15 years earlier, got in touch with Leslie Gordon. "Francis called me and said, Hey, I just saw a talk by David Liu from the Broad [Institute]. And it was pretty amazing. He has been looking at progeria and has very early, but very exciting data… Do you have any spaces, any slots you could make in your program for late breaking news?"
Gordon found a spot, and David Liu came to talk about what was going on in his lab, which was an even more advanced treatment that led to mice with the progeria mutation living into their senior mouse years—substantially closer to a normal lifespan. Liu's lab had built on the idea of CRISPR-Cas9 to create a more elegant genetic process called base editing: Instead of chopping out mutated DNA, a scientist could chemically convert an incorrect DNA letter to the correct one, like the search and replace function in word processing software. Mice who had their Lamin-A mutations corrected this way lived more than twice as long as untreated animals.
Sammy was in the audience at Dr. Liu's talk. "When I heard about this base editing as a younger scientist, I thought that I was living in the future," he says. "When my parents had my diagnosis of progeria, the science knew very little information about DNA. And now we are talking about healing the DNA… It is incredible."
Lonafarnib (also called Zokinvy) was approved by the US Food and Drug Administration this past November. Sammy, now 25, still takes it, and still manages his side effects. With luck, the gift of a few extra years will act as a bridge until he can try Liu's revolutionary new gene treatment, which has not yet begun testing in humans. While Leslie Gordon warns that she's always wrong about things like this, she hopes to see the new base editing techniques in clinical trials in the next year or two. Sammy won't need to be convinced to try it this time; his thinking on fate has evolved since his first encounter with lonafarnib.
"I would be very happy to try it," he says. "I know that for a non-scientist it can be difficult to understand. Some people think that we are the DNA. We are not. The DNA is a part of us, and to correct it is to do what we are already doing—just better." In short, a gene therapy, while it may seem like science fiction, is no different from a pill. For Sammy, both are a new way to think about fate: No longer something that simply happens to him.
Virtual clinical trials are helping to eradicate logistical barriers to clinical trial participation, though trust is still an issue.
Herman Taylor, director of the cardiovascular research institute at Morehouse college, got in touch with UnitedHealth Group early in the pandemic.
The very people who most require solutions to COVID are those who are least likely to be involved in the search to find them.
A colleague he worked with at Grady Hospital in Atlanta was the guy when it came to studying sickle cell disease, a recessive genetic disorder that causes red blood cells to harden into half-moon shapes, causing cardiovascular problems. Sickle cell disease is more common in African Americans than it is in Caucasians, in part because having just one gene for the disease, called sickle cell trait, is protective against malaria, which is endemic to much of Africa. Roughly one in 12 African Americans carry sickle cell trait, and Taylor's colleague wondered if this could be one factor affecting differential outcomes for COVID-19.
UnitedHealth Group granted Taylor and his colleague the money to study sickle cell trait in COVID, and then, as they continued working together, they began to ask Taylor his opinion on other topics. As an insurance company, United had realized early in the pandemic that it was sitting on a goldmine of patient data—some 120 million patients' worth—that it could sift through to look for potential COVID treatments.
Their researchers thought they had found one: In a small subset of 14,000 people who'd contracted COVID, there was a group whose bills were paid by Medicare (which the researchers took as a proxy for older age). The people in this group who were taking ACE inhibitors, blood vessel dilators often used to treat high blood pressure, were 40 percent less likely to be hospitalized than those who were not taking the drug.
The connection between ACE inhibitors and COVID hospitalizations was a correlation, a statistical association. To determine whether the drugs had any real effect on COVID outcomes, United would have to perform another, more rigorous study. They would have to assign some people to receive small doses of ACE inhibitors, and others to receive placebos, and measure the outcomes under each condition. They planned to do this virtually, allowing study participants to sign up and be screened online, and sending drugs, thermometers, and tests through the mail. There were two reasons to do it this way: First, the U.S. Food and Drug Administration had been advising medical researchers to embrace new strategies in clinical trials as a way to protect participants during the pandemic.
The second reason was why they asked Herman Taylor to co-supervise it: Clinical trials have long had a diversity problem. And going virtual is a potential solution.
Since the beginning of the pandemic, COVID-19 has infected people of color at a rate of three times that of Caucasians (killing Black people at a rate 2.5 times as high, and Hispanic and American Indian or Alaska Native people at a rate 1.3 times as high). A number of explanations have been put forth to explain this disproportionate toll. Among them: higher levels of poverty, essential jobs that increase exposure, and lower quality or inadequate access to medical care.
Unfortunately, these same factors also affect who participates in research. People of color may be less likely to have doctors recommend studies to them. They may not have the time or the resources to hang out in a waiting room for hours. They may not live near large research institutions that conduct trials. The result is that new treatments, even for diseases that affect Latin, Native American, or African American populations in greater proportions, are studied mostly in white volunteers. The very people who most require solutions to COVID are those who are least likely to be involved in the search to find them.
Virtual trials can alleviate a number of these problems. Not only can people find and request to participate in these types of trials through their phones or computers, virtual trials also cover more costs, include a larger geographical range, and have inherently flexible hours.
"[In a traditional study] you have to go to a doctor's office to enroll and drive a couple of hours and pay $20 for parking and pay $15 for a sandwich in the hospital cafeteria and arrange for daycare for your kids and take time off of work," says Dr. Jonathan Cotliar, chief medical officer of Science37, a platform that investigators can hire to host and organize their trials virtually. "That's a lot just for one visit, much less over the course of a six to 12-month study."
Cotliar's data suggests that virtual trials' enhanced access seriously affects the racial makeup of a given study's participant pool. Sixty percent of patients enrolled in Science37 trials are non-Caucasian, which is, Cotliar says, "staggering compared to what you find in traditional site-based research."
But access is not the only barrier to including more people of color in clinical trials. There is also trust. When agreeing to sign up for research, undocumented immigrants may worry about finding themselves in legal trouble or without any medical support should something go wrong. In a country with a history of experimenting on African Americans without their consent, black people may not trust institutions not to use them as guinea pigs.
"A lot of people report being somewhat disregarded or disrespected once entering the healthcare system," Taylor says. "You take it all together, then people wonder, well, okay, this is how the system tends to regard me, yet now here come these people doing research, and they're all about getting me into their studies." Not so surprising that a lot of people may respond with a resounding "No thanks."
United's ACE inhibitor trial was notable for addressing both of these challenges. In addition to covering costs and allowing study subjects to participate from their own homes, it was being co-sponsored by a professor at Morehouse, one of the country's historic black colleges and universities (often abbreviated HBCUs). United was recruiting heavily in Atlanta, whose population is 52 percent African American. The study promised a thoughtful introduction to a more egalitarian future of medical research.
There's just one problem: It isn't going to happen.
This month, in preparation for the study, United reanalyzed their ACE inhibitor data with all the new patients who'd contracted COVID in the months since their first analysis. Their original data set had been concentrated in the Northeast, mostly New York City, where the earliest outbreak took place. In the 12 weeks it had taken them to set up the virtual followup study, epicenters had shifted. United's second, more geographically comprehensive sample had ten times the number of people in it. And in that sample, the signal simply disappeared.
"I was shocked, but that's the reality," says Deneen Vojta, executive vice president of enterprise research and development for UnitedHealth Group. "You make decisions based on the data, but when you get more data, more information, you might make a different decision. The answer is the answer."
There was no point in running a virtual ACE inhibitor study if a larger, more representative sample of people indicated the drug was unlikely to help anyone. Still, the model United had established to run the trial remains viable. Even as she scrapped the ACE inhibitor study, Vojta hoped not just to continue United's relationship with Dr. Taylor and Morehouse, but to formalize it. Virtual platforms are still an important part of their forthcoming trials.
If people don't believe a vaccine has been created with them in mind, then they won't take it, and it won't matter whether it exists or not.
United is not alone in this approach. As phase three trials for vaccines against SARS CoV-2 get underway, big pharma companies have been publicly articulating their own strategies for including more people of color in clinical trials, and many of these include virtual elements. Janelle Sabo, global head of clinical innovation, systems and clinical supply chain at Eli Lilly, told me that the company is employing home health and telemedicine, direct-to-patient shipping and delivery, and recruitment using social media and geolocation to expand access to more diverse populations.
Dr. Macaya Douoguih, Head of Clinical Development and Medical Affairs for Janssen Vaccines under Johnson & Johnson, spoke to Congress about this issue during a July hearing before the House Energy and Commerce Oversight and Investigations Subcommittee. She said that the company planned to institute a "focused digital and community outreach plan to provide resources and opportunities to encourage participation in our clinical trials," and had partnered with Johns Hopkins Bloomberg School of Public Health "to understand how the COVID-19 crisis is affecting different communities in the United States."
But while some of these plans are well thought-out, others are concerningly nebulous, featuring big pronouncements but fewer tangible strategies. In that same July hearing, Massachusetts representative Joe Kennedy III (D) sounded like a frustrated teacher when admonishing four of the five leads of the present pharma companies (AstraZeneca, Johnson & Johnson, Merck, Moderna, and Pfizer) for not explaining exactly how they'd ensure diversity both in the study of their vaccines, and in their eventual distribution.
This matters: The uptake of the flu vaccine is 10 percentage points lower in both the African American and Hispanic communities than it is in Caucasians. A Pew research study conducted early in the pandemic found that just 54 percent of Black adults said they "would definitely or probably get a coronavirus vaccine," compared to 74 percent of Whites and Hispanics.
"As a good friend of mine, Dr. [James] Hildreth, president at Meharry, another HBC medical school, likes to say: 'A vaccine is great, but it is the vaccination that saves people,'" Taylor says. If people don't believe a vaccine has been created with them in mind, then they won't take it, and it won't matter whether it exists or not.
In this respect, virtual platforms remain an important first step, at least in expanding admittance. In June, United Health opened up a trial to their entire workforce for a computer game that could treat ADHD. In less than two months, 1,743 people had signed up for it, from all different socioeconomic groups, from all over the country. It was inching closer to the kind of number you need for a phase three vaccine trial, which can require tens of thousands of people. Back when they'd been planning the ACE inhibitor study, United had wanted 9,600 people to agree to participate.
Now, with the help of virtual enrollment, they hope they can pull off similarly high numbers for the COVID vaccine trial they will be running for an as-yet-unnamed pharmaceutical partner. It stands to open in September.