Jamie Metzl, author of Hacking Darwin, shares his views with Leaps.org on the future of genetics, tech, healthcare and more.
In this Q&A, leading technology and healthcare futurist Jamie Metzl discusses a range of topics and trend lines that will unfold over the next several decades: whether a version of Moore's Law applies to genetic technologies, the ethics of genetic engineering, the dangers of gene hacking, the end of sex, and much more.
Metzl is a member of the WHO expert advisory committee on human genome editing and the bestselling author of Hacking Darwin.
The conversation was lightly edited by Leaps.org for style and length.
In Hacking Darwin, you describe how we may modify the human body with CRISPR technologies, initially to obtain unsurpassed sports performance and then to enhance other human characteristics. What would such power over human biology mean for the future of our civilization?
After nearly four billion years of evolution, our one species suddenly has the increasing ability to read, write, and hack the code of life. This will have massive implications across the board, including in human health and reproduction, plant and animal agriculture, energy and advanced materials, and data storage and computing, just to name a few. My book Hacking Darwin: Genetic Engineering and the Future of Humanity primarly explored how we are currently deploying and will increasingly use our capabilities to transform human life in novel ways. My next book, The Great Biohack: Recasting Life in an Age of Revolutionary Technology, coming out in May 2024, will examine the broader implications for all of life on Earth.
We humans will, over time, use these technologies on ourselves to solve problems and eventually to enhance our capabilities. We need to be extremely conservative, cautious, and careful in doing so, but doing so will almost certainly be part of our future as a species.

In electronics, Moore's law is an established theory that computing power doubles every 18 months. Is there any parallel to be drawn with genetic technologies?
The increase in speed and decrease in costs of genome sequencing have progressed far faster than Moore’s law. It took thirteen years and cost about a billion dollars to sequence the first human genome. Today it takes just a few hours and can cost as little as a hundred dollars to do a far better job. In 2012, Jennifer Doudna and Emmanuel Charpentier published the basic science paper outlining the CRISPR-cas9 genome editing tool that would eventually win them the Nobel prize. Only six years later, the first CRISPR babies were born in China. If it feels like technology is moving ever-faster, that’s because it is.
Let's turn to the topic of aging. Do you think that the field of genetics will advance fast enough to eventually increase maximal lifespan for a child born this year? How about for a person who is currently age 50?
The science of aging is definitely real, but that doesn’t mean we will live forever. Aging is a biological process subject to human manipulation. Decades of animal research shows that. This does not mean we will live forever, but it does me we will be able to do more to expand our healthspans, the period of our lives where we are able to live most vigorously.
The first thing we need to do is make sure everyone on earth has access to the resources necessary to live up to their potential. I live in New York City, and I can take a ten minute subway ride to a neighborhood where the average lifespan is over a decade shorter than in mine. This is true within societies and between countries as well. Secondly, we all can live more like people in the Blue Zones, parts of the world where people live longer, on average, than the rest of us. They get regular exercise, eat healthy foods, have strong social connections, etc. Finally, we will all benefit, over time, from more scientific interventions to extend our healthspan. This may include small molecule drugs like metformin, rapamycin, and NAD+ boosters, blood serum infusions, and many other things.
Science fiction has depicted a future where we will never get sick again, stay young longer or become immortal. Assuming that any of this is remotely possible, should we be afraid of such changes, even if they seem positive in some regards, because we can’t understand the full implications at this point?
Not all of these promises will be realized in full, but we will use these technologies to help us live healthier, longer lives. We will never become immortal becasue nothing lasts forever. We will always get sick, even if the balance of diseases we face shifts over time, as it has always done. It is healthy, and absolutely necessary, that we feel both hope and fear about this future. If we only feel hope, we will blind ourselves to the very real potential downsides. If we only feel fear, we will deny ourselves the very meaningful benefits these technologies have the potential to provide.
A fascinating chapter in Hacking Darwin is entitled The End of Sex. And you see that as a good thing?
We humans will always be a sexually reproducing species, it’s just that we’ll reproduce increasingly less through the physical act of sex. We’re already seeing this with IVF. As the benefits of technology assisted reproduction increase relative to reproduction through the act of sex, many people will come to see assisted reproduction as a better way to reduce risk and, over time, possibly increase benefits. We’ll still have sex for all the other wonderful reasons we have it today, just less for reproduction. There will always be a critical place in our world for Italian romantics!
What are dangers of genetic hackers, perhaps especially if everyone’s DNA is eventually transcribed for medical purposes and available on the internet and in the cloud?
The sky is really the limit for how we can use gentic technologies to do things we may want, and the sky is also the limit for potential harms. It’s quite easy to imagine scenarios in which malevolent actors create synthetic pathogens designed to wreak havoc, or where people steal and abuse other people’s genetic information. It wouldn’t even need to be malevolent actors. Even well-intentioned researchers making unintended mistakes could cause real harm, as we may have seen with COVID-19 if, as appears likely to me, the pandemic stems for a research related incident]. That’s why we need strong governance and regulatory systems to optimize benefits and minimize potential harms. I was honored to have served on the World Health Organization Expert Advisory Committee on Human Genome Editing, were we developed a proposed framework for how this might best be achieved.
You foresee the equivalent of a genetic arms race between the world's most powerful countries. In what sense are genetic technologies similar to weapons?
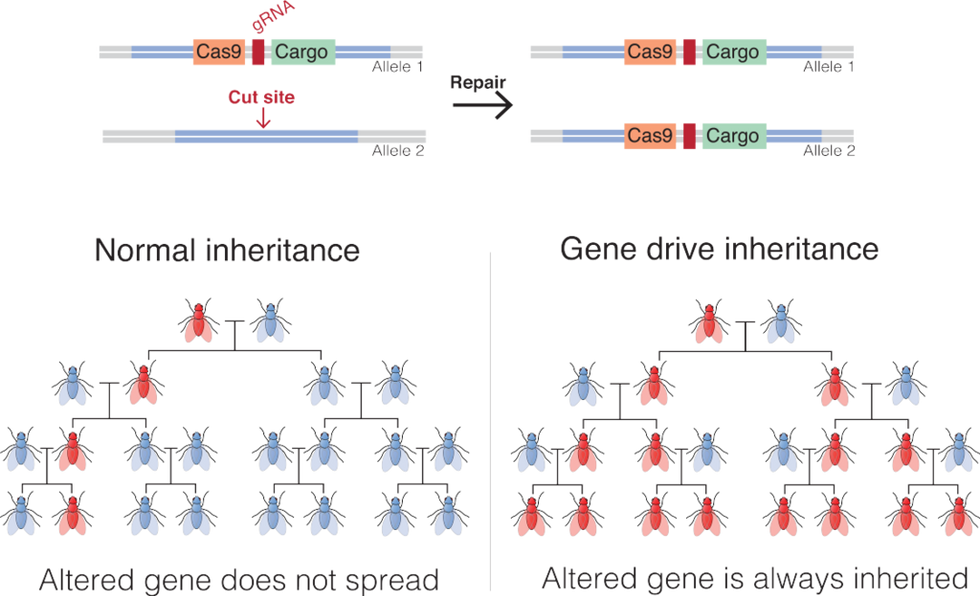
Genetic technologies could be used to create incredibly powerful bioweapons or to build gene drives with the potential to crash entire ecosystems. That’s why thoughtful regulation is in order. Because the benefits of mastering and deploying these technologies are so great, there’s also a real danger of a genetics arms race. This could be extremely dangerous and will need to be prevented.
In your book, you express concern that states lacking Western conceptions of human rights are especially prone to misusing the science of genetics. Does this same concern apply to private companies? How much can we trust them to control and wield these technologies?
This is a conversation about science and technology but it’s really a conversation about values. If we don’t agree on what core values should be promoted, it will be nearly impossible to agree on what actions do and do not make sense. We need norms, laws, and values frameworks that apply to everyone, including governments, corporations, researchers, healthcare providers, DiY bio hobbyists, and everyone else.
We have co-evolved with our technology for a very long time. Many of our deepest beliefs have formed in that context and will continue to do so. But as we take for ourselves the powers we have attributed to our various gods, many of these beliefs will be challenged. We can not and must not jettison our beliefs in the face of technology, and must instead make sure our most cherished values guide the application of our most powerful technologies.
A conversation on international norms is in full swing in the field of AI, prompted by the release of ChatGPT4 earlier this year. Are there ways in which it’s inefficient, shortsighted or otherwise problematic for these discussions on gene technologies, AI and other advances to be occurring in silos? In addition to more specific guidelines, is there something to be gained from developing a universal set of norms and values that applies more broadly to all innovation?
AI is yet another technology where the potential to do great good is tied to the potential to inflict signifcant harm. It makes no sense that we tend to treat each technology on its own rather than looking at the entire category of challenges. For sure, we need to very rapidly ramp up our efforts with regard to AI norm-setting, regulations, and governance at all levels. But just doing that will be kind of like generating a flu vaccine for each individual flu strain. Far better to build a universal flu vaccine addressing common elements of all flu viruses of concern.
That’s why we also need to be far more deliberate in both building a global operating systems based around the mutual responsibilities of our global interdependence and, under that umbrella, a broader system for helping us govern and regulate revolutionary technologies. Such a process might begin with a large international conference, the equivalent of Rio 1992 for climate change, but then quickly work to establish and share best practices, help build parallel institutions in all countries so people and governamts can talk with each other, and do everything possible to maximize benefits and minimize risks at all levels in an ongoing and dynamic way.
At what point might genetic enhancements lead to a reclassfication of modified humans as another species?
We’ll still all be fellow humans for a very, very long time. We already have lots of variation between us. That is the essence of biology. Will some humans, at some point in the future, leave Earth and spend generations elsewhere? I believe so. In those new environments, humans will evolve, over time, differently than those if us who remain on this planet? This may sound like science fiction, but the sci-fi future is coming at us faster than most people realize.
Is the concept of human being changing?
Yes. It always has and always will.
Another big question raised in your book: what limits should we impose on the freedom to manipulate genetics?
Different societies will come to different conclusion on this critical question. I am sympathetic to the argument that people should have lots of say over their own bodies, which why I support abortion rights even though I recognize that an abortion can be a violent procedure. But it would be insane and self-defeating to say that individuals have an unlimited right to manipulate their own or their future children’s heritable genetics. The future of human life is all of our concern and must be regulated, albeit wisely.
In some cases, such as when we have the ability to prevent a deadly genetic disroder, it might be highly ethical to manipulate other human beings. In other circumstances, the genetic engineering of humans might be highly unethical. The key point is to avoid asking this question in a binary manner. We need to weigh the costs and benefits of each type of intervention. We need societal and global infrastrucutres to do that well. We don’t yet have those but we need them badly.
Can you tell us more about your next book?
The Great Biohack: Recasting Lifee in an Age of Revolutionary Technology, will come out in May 2024. It explores what the intersecting AI, genetics, and biotechnology revolutions will mean for the future of life on earth, including our healthcare, agriculture, industry, computing, and everything else. We are at a transitional moment for life on earth, equivalent to the dawn of agriculture, electricity, and industrialization. The key differentiator between better and worse outcomes is what we do today, at this early stage of this new transformation. The book describes what’s happening, what’s at stake, and what we each and all can and, frankly, must do to build the type of future we’d like to inhabit.
You’ve been a leader of international efforts calling for a full investigation into COVID-19 origins and are the founder of the global movement OneShared.World. What problem are you trying to solve through OneShared.World?
The biggest challenge we face today is the mismatch between the nature of our biggest problems, global and common, and the absence of a sufficient framework for addressing that entire category of challenges. The totally avoidable COVID-19 pandemic is one example of the extremet costs of the status quo. OneShared.World is our effort to fight for an upgrade in our world’s global operating system, based around the mutual responsibilities of interdependence. We’ve had global OS upgrades before after the Thirty Years War and after World War II, but wouldn’t it be better to make the necessary changes now to prevent a crisis of that level stemming from a nuclear war, ecosystem collapse, or deadlier synthetic biology pandemic rather than waiting until after? Revolutionary science is a global issue that must be wisely managed at every level if it is to be wisely managed at all.
How do we ensure that revolutionary technologies benefit humanity instead of undermining it?
That is the essential question. It’s why I’ve written Hacking Darwin, am writing The Great Biohack, and doing the rest of my work. If we want scietific revolutions to help, rather than hurt, us, we must all play a role building that future. This isn’t just a conversation about science, it’s about how we can draw on our most cherished values to guide the optimal development of science and technology for the common good. That must be everyone’s business.
Portions of this interview were first published in Grassia (Italy) and Zen Portugal.
Jamie Metzl is one of the world’s leading technology and healthcare futurists and author of the bestselling book, Hacking Darwin: Genetic Engineering and the Future of Humanity, which has been translated into 15 languages. In 2019, he was appointed to the World Health Organization expert advisory committee on human genome editing. Jamie is a faculty member of Singularity University and NextMed Health, a Senior Fellow of the Atlantic Council, and Founder and Chair of the global social movement, OneShared.World.
Called “the original COVID-19 whistleblower,” his pioneering role advocating for a full investigation into the origins of the COVID-19 pandemic has been featured in 60 Minutes, the New York Times, and most major media across the globe, and he was the lead witness in the first congressional hearings on this topic. Jamie previously served in the U.S. National Security Council, State Department, and Senate Foreign Relations Committee and with the United Nations in Cambodia. Jamie appears regularly on national and international media and his syndicated columns and other writing in science, technology, and global affairs are featured in publications around the world.
Jamie sits on advisory boards for multiple biotechnology and other companies and is Special Strategist to the WisdomTree BioRevolution Exchange Traded Fund. In addition to Hacking Darwin, he is author of a history of the Cambodian genocide, the historical novel The Depths of the Sea, and the genetics sci-fi thrillers Genesis Code and Eternal Sonata. His next book, The Great Biohack: Recasting Life in an age of Revolutionary Technology, will be published by Hachette in May 2024. Jamie holds a Ph.D. from Oxford, a law degree from Harvard, and an undergraduate degree from Brown and is an avid ironman triathlete and ultramarathon runner.