How Bacteria-Killing Viruses May Save Us From Antibiotic Resistance
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
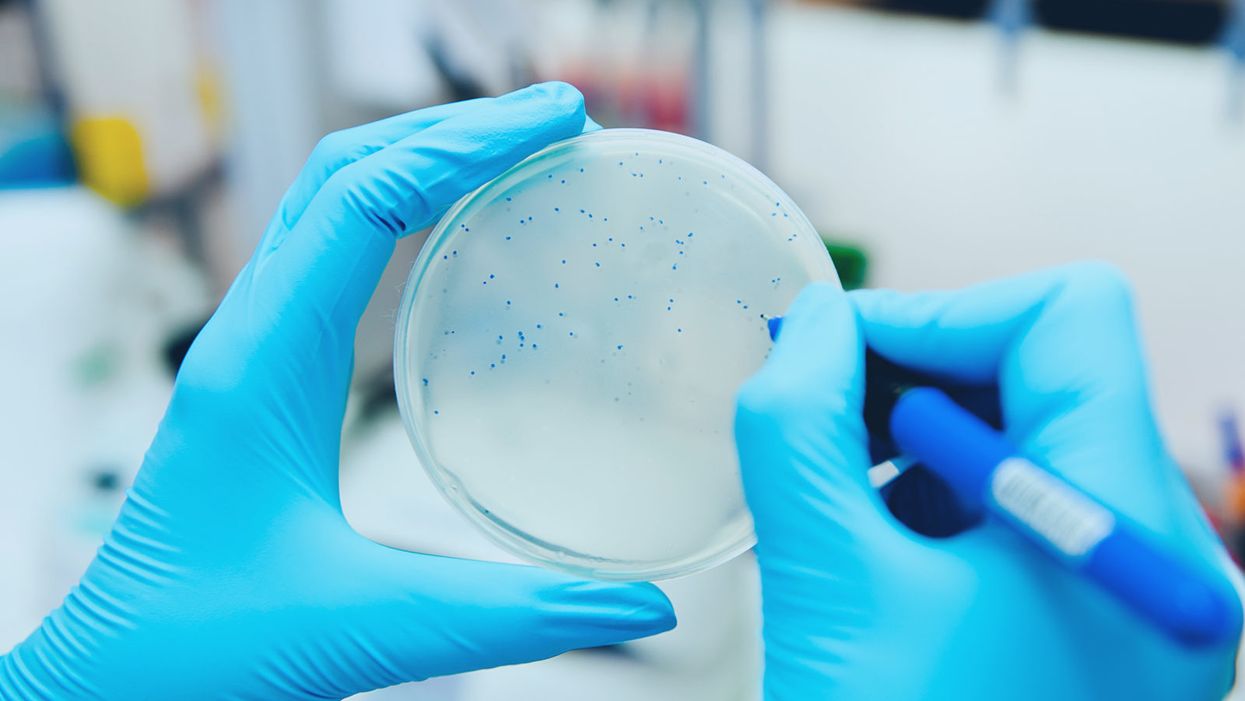
Hand-counting bacteriophage plaques during a titer test.
In my hometown of Pittsburgh, it is not uncommon to read about cutting-edge medical breakthroughs, because Pittsburgh is the home of many innovations in medical science, from the polio vaccine to pioneering organ transplantation. However, medical headlines from Pittsburgh last November weren't heralding a new discovery for once. They were carrying a plea—for a virus.
Phages are weapons of bacterial destruction, but despite recognition of their therapeutic potential for over 100 years, there are zero phage products commercially available to medicine in the United States.
Specifically, a bacteria-killing virus that could attack and control a certain highly drug-resistant bacterial infection ravaging the newly transplanted lungs of a 25-year-old woman named Mallory Smith. The culprit bacteria, Burkholderia cepacia, is a notoriously vicious bacterium that preys on patients with cystic fibrosis who, throughout their life, are exposed to course after course of antibiotics, often fostering a population of highly resistant bacteria that can become too formidable for modern medicine to combat.
What Smith and her physicians desperately needed was a tool that would move beyond failed courses of antibiotics. What they sought was called a bacteriophage. These are naturally occurring ubiquitous viruses that target not humans, but bacteria. The world literally teems with "phages" and one cannot take a bite or drink of anything without encountering them. These weapons of bacterial destruction are exquisitely evolved to target bacteria and, as such, are not harmful to humans. However, despite recognition of their therapeutic potential for over 100 years, there are zero bacteriophage products commercially available to medicine in the United States, at a time when antibiotic resistance is arguably our most pressing public health crisis. Just this week, a new study was published in the Proceedings of the National Academy of Sciences detailing the global scope of the problem.
Why Were These Promising Tools Forgotten?
Phages weren't always relegated to this status. In fact, in the early 20th century phages could be found on American drug store shelves and were used for a variety of ailments. However, the path-breaking discovery and development of antimicrobials agents such as the sulfa drugs and, later the antibiotic penicillin, supplanted the world of phage therapeutics in the United States and many other places.
Fortunately, phage therapy never fully disappeared, and research and clinical use continued in Eastern European nations such as Georgia and Poland.
The antibiotic age revolutionized medicine in a way that arguably no other innovation has. Not only did antibiotics tame many once-deadly infectious diseases, but they made much of modern medicine – from cancer chemotherapy to organ transplantation to joint replacement – possible. Antibiotics, unlike the exquisitely evolved bacteriophage, possessed a broader spectrum of activity and were active against a range of bacteria. This non-specificity facilitated antibiotic use without the need for a specific diagnosis. A physician does not need to know the specific bacterial genus and species causing, for example, a skin infection or pneumonia, but can select an antibiotic that covers the likely culprits and use it empirically, fully expecting the infection to be controlled. Unfortunately, this non-specificity engendered the overuse of antibiotics whose consequences we are now suffering. A bacteriophage, on the other hand, will work against one specific bacterial species and is evolved for just that role.
Phages to the Rescue
As the march of antibiotic resistance has predictably continued since the dawn of the antibiotic age, the prospect of resurrecting phage therapy has been increasingly viewed as one solution. Fortunately, phage therapy never fully disappeared, and research and clinical use continued in Eastern European nations such as Georgia and Poland. However, much of that experience has remained opaque to the medical community at large and questions about dosage, toxicity, efficacy, and method of delivery left many questions without full answers.
Though real questions remained regarding phage use, dire circumstances of prolific antibiotic resistance necessitated their use in the U.S. in two prominent instances involving life-threatening infections. The first case involved an Acinetobacter baumanii infection of the pancreas in a San Diego man in which phages were administered intravenously in 2016. The other case, also in 2016, involved the instillation of phages, fished out of a pond, into the chest cavity of man with a Pseudmonas aeruginosa infection of a prosthetic graft of the aorta. Both cases were successful and were what fueled the Pittsburgh-based plea for Burkholderia phages.
The phages you begin with may not be the ones you end up with, as Darwinian evolutionary pressures will alter the phage in order to keep up with the ongoing evolution of its bacterial target.
How Phages Differ from Other Medical Products
It might seem surprising that in light of the urgent need for new treatments for drug-resistant infections, the pharmaceutical armamentarium is not teeming with phages like a backyard pond. However, phages have been difficult to fit into the current regulatory framework that operates in most developed countries such as the U.S. because of their unique characteristics.
Phages are not one homogenous product like a tablet of penicillin, but a cocktail of viruses that change and evolve as they replicate. The phages you begin with may not be the ones you end up with, as Darwinian evolutionary pressures will alter the phage in order to keep up with the ongoing evolution of its bacterial target. The cocktail may not just contain one specific phage, but a range of phages that all target some specific bacteria in order to increase efficacy. These phage cocktails might also need adjusting to keep pace with bacterial resistance. Additionally, the concentration of phage in a human body after administration is not so easy to predict as phage numbers will rise and fall based on the number of target bacteria that are present.
All of these characteristics make phages very unique when viewed through a regulatory lens, and necessitate the creation of new methods to evaluate them, given that regulatory approval is required. Using phages in the U.S. now requires FDA permission through an investigational new drug application, which can be expedited during an emergency situation. FDA scientists are actively involved in understanding the best means to evaluate bacteriophage therapy and several companies are in early-stage development, though no major clinical trials in the U.S. are currently underway.
One FDA-approved application of phages has seen them used on food products at delis and even in slaughterhouses to diminish the quantity of bacteria on certain meat products.
Would That Humans Were As Lucky As Bologna
Because of the regulatory difficulties with human-use approval, some phage companies have taken another route to develop phage products: food safety. Food safety is a major public health endeavor, and keeping food that people consume safe from E.coli, Listeria, and Salmonella, for example, are rightfully major priorities of industry. One FDA-approved application of phages has seen them used on food products at delis and even in slaughterhouses to diminish the quantity of bacteria on certain meat products.
This use, unlike that for human therapeutic purposes, has found success with regulators: phages, not surprisingly, have been granted the "generally regarded as safe (GRAS)" designation.
A Phage Directory
Tragically Mallory Smith succumbed to her infection despite getting a dose of phages culled from sludge in the Philippines and Fiji. However, her death and last-minute crusade to obtain phages has prompted the call for a phage directory. This directory could catalog the various phages being studied and the particular bacteria they target. Such a searchable index will facilitate the rapid identification and – hopefully – delivery of phages to patients.
If phage therapy is to move from a last-ditch emergency measure to a routine tool for infectious disease physicians, it will be essential that the hurdles they face are eliminated.
Moving Beyond Antibiotics
As we move increasingly toward a post-antibiotic age in infectious disease, moving outside of the traditional paradigm of broad-spectrum antibiotics to non-traditional therapeutics such as bacteriophages and other novel products will become increasingly necessary. Already, clinical trials are underway in various populations, including a major trial in European burn patients.
It is important to understand that there are important scientific and therapeutic questions regarding dose, route of administration and other related questions that need to be addressed before phage use becomes more routine, and it is only through clinical trials conducted with the hope of eventual commercialization that these answers will be found. If phage therapy is to move from a last-ditch emergency measure to a routine tool for infectious disease physicians, it will be essential that the hurdles they face are eliminated.
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”

